Unfortunately, these highly-specialized cells don’t naturally regenerate, so hearing loss is permanent. Research on inner ear hair cell regeneration has expanded recently and shows promise for future therapeutic options.
Researchers at the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at Keck Medicine of USC are studying the embryonic development of the hair cells to better understand their failure to regenerate. A number of years ago, these researchers discovered that a latent potential for regeneration existed in newborn mice, not unlike that which exists in non-mammalian vertebrates like birds and reptiles, but that this latent potential is rapidly lost during mouse postnatal maturation. Their leading hypothesis to explain this loss is that epigenetic change (change to the structure of the DNA and its associated proteins) is responsible for blocking the future ability to regenerate. They are now investigating methods for manipulating the epigenetic state of these cells to allow regeneration to occur.
One of the major obstacles in research to understand the problems of hearing loss is the lack of sufficient numbers of sensory hair cells to study. The human inner ear has only about 10,000 hair cells. Similarly, only a few thousand cells are available from a mouse inner ear, and these are lodged in the temporal bone, making their extraction difficult. To overcome this limitation, researchers in the Segil and Ichida labs in the Eli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research at USC are learning how to generate large quantities of hair cells in the lab using a technique called direct lineage reprogramming. In these studies, large numbers of hair cells are generated artificially in vitro from mouse and human somatic cells (skin cells or fibroblasts). Once the hair cells are generated, researchers characterize the cellular and molecular biology of the induced hair cells (see Figure 1).
Investigators are using these artificial hair cells to screen for new therapeutic compounds that can block the ototoxic effects of useful drugs, such as certain antibiotics and chemotherapy agents, and stimulate regeneration. The technique of direct lineage reprogramming also allows the researchers to model genetically-linked forms of hearing loss by engineering mutations that mimic naturally occurring hearing loss genes, such as the gene for autosomal dominant deafness (DFNA2a), which is caused by a mutation in the channel protein KCNQ4.
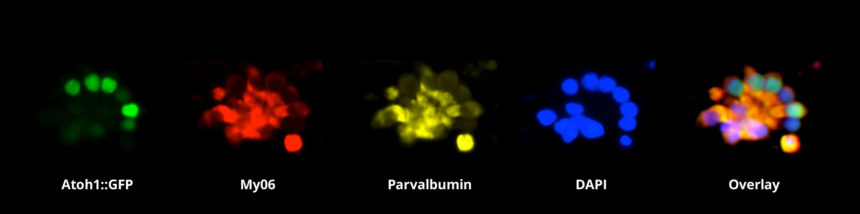
Figure 1 – Immunohistochemical staining of sensory hair cell-like cells. The cells were induced from fibroblasts by the over-expression of selected transcription factors. Image courtesy of Suhasni Gopalakrishnan, Louise Menendez, Juan Llamas, Justin Ichida and Neil Segil.